Mechanism of arrhythmia
Automaticity
Altered normal automaticity
Structures that possess the ability to depolarization spontaneously:
-
Sinoatrial node: The SA node is the primary pacemaker of the heart.
-
Parts of atrial myocardium: There are clusters of myocardium that possess automaticity, e.g., around crista terminalis, entrance of coronary sinus and inferior vena cava, around mitral and tricuspid valve.
-
Myocardium surrounding atrioventricular (AV) node: Cell clusters around the AV node also possess automaticity.
-
His-Purkinje system: The Bundle of his and Purkinje system possess automaticity.
The intrinsic rate of spontaneous depolarization:
-
Sinoatrial node 70/min
-
Atrial myocardium 60/min
-
Myocardium surrounding AV node 40/min
-
His-Purkinje system: 20-40/min
Under normal conditions, the sinoatrial nodal cells fire at the fastest rate, while the "subsidiary" pacemaker cells fire at a slower rate.
Sinus node action potential

muscle action potential

The firing rate of the pacemaker is determined by the interaction of 3 factors:
e.g. increase sympathetic acitivity, hypoxia (enhancing phase 4 diastolic deploarization) --> increase rate
e.g. increase vagal tone--> slower rate



Catecholamines enhance the permeability of Ica-L, leading to an increased ICa2+ current. Additionally, sympathetic activity boosts the If current, raising the phase 4 repolarization slope.
Metabolic abnormalities like hypoxia and hypokalemia can increase normal automatic activity due to Na/K pump inhibition, which reduces the repolarizing current and enhances phase 4 diastolic repolarization.
Parasympathetic activity decreases the discharge rate of pacemaker cells by releasing acetylcholine (Ach) and hyperpolarizing the cells by enhancing K+ conductance. Furthermore, it may diminish ICa-L and If activity, leading to an additional reduction in the rate.
Altered normal automaticity is likely sensitive to overdrive suppression.
Abnormal automaticity
Structures in the heart that normally cannot depolarize spontaneously:
This can occur under conditions that push the maximum diastolic potential closer to the threshold potential. This phenomenon is explained by the interaction of various currents that collectively produce a net inward depolarizing current, which is linked to a reduction in potassium conductance.
The intrinsic rate of an automatic abnormal focus depends on the membrane potential; the more positive the membrane potential, the higher the automatic rate.
Example: Premature beats, atrial tachycardia, accelerated idioventricular rhythm, ventricular tachycardia (VT), particularly in the acute phase, associated with ischemia and reperfusion.
Re-entry
Anatomical re-entry:
an inexcitable anatomical obstacle encircled by a circular pathway where the wavefront can "reenter," forming fixed and stable reentrant circuits.

Slow pathway
(shorter refractory period)
Fast pathway
(longer refractory period)
Entry
Exit
Central area of block
Head: Wave-front propagation
Tail: Refractory period
A normal impulse arriving at the entry goes down both the slow and fast pathways. Conduction through the slow path is slower, while conduction through the fast pathway is quicker. The fast pathway brings the signals to the ventricle. In addition to stimulating normal ventricular depolarization, it also retraces back up the slow path. When this retracing signal meets the signal from the slow pathway, they cancel each other out.
Unidirectional block
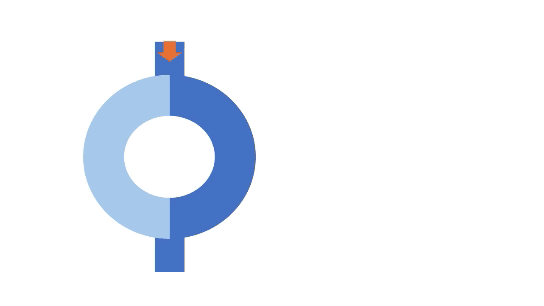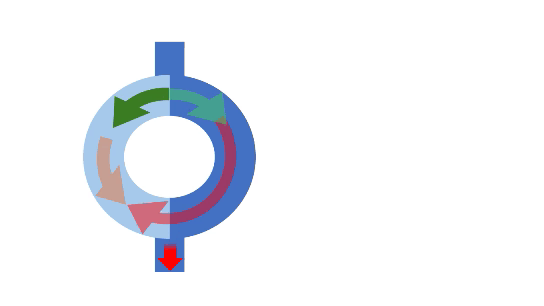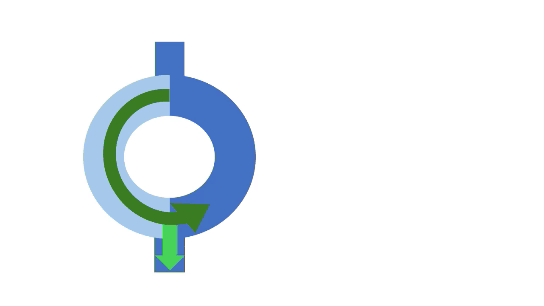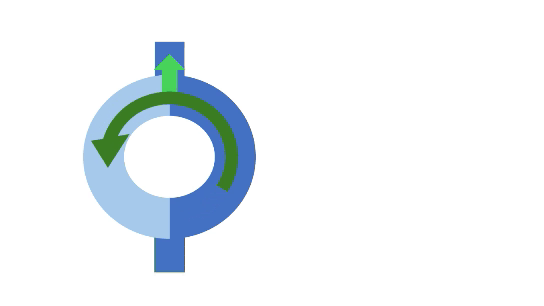
Extrasystole
Central area of block
Entry
Exit
Slow pathway
(shorter refractory period)
Fast pathway
(longer refractory period)
Unidirectional block occur here
Head: Wave-front propagation
Tail: Refractory period
Excitable gap
In the reentrance mechanism, an extrasystolic impulse enters the node, which features two distinct pathways: the “fast” and “slow” pathways. With its longer refractory period, the fast pathway effectively blocks the incoming impulse, creating a unidirectional block, while the slow pathway conducts normally. The fast pathway recovers as the impulse reaches the distal junction and travels back up, allowing for retrograde conduction. This process establishes a reentrant circuit that perpetuates the tachycardia.
Functional Reentry:
Anatomical obstacles do not define the circuit; it is shaped by dynamic heterogeneities in the electrophysiological properties of the involved tissue.
e.g., myocardial ischemia, infarction related-VT, presence of severely impaired conduction, Spiral wave (rotor) activity (atrial and ventricular fibrillation, polymorphic VT.)
Trigger activity(TA)
Triggered activity (TA) refers to impulse initiation that occurs due to afterdepolarizations.
Afterdepolarizations only occur after a preceding action potential (the trigger), and a new action potential is generated when they achieve the threshold potential.

Phase 2 early after depolarization
Phase 3 early after depolarization
QT prolong due to various causes.
Delayed after depolarization
Death (myocardium): post-MI
Adrenergic: CPVT, catecholamines
Digoxin
Delayed After depolarization
A DAD is an oscillation in membrane voltage that occurs after repolarization of the action potential (during phase 4).
It is commonly caused by various conditions that raise the diastolic intracellular Ca2+ concentration.
Digoxin inhibits the Na/K ATPase, facilitating the Na+/Ca2+ exchanger and increasing intracellular calcium concentration. Clinically, digoxin toxicity leading to bidirectional fascicular tachycardia is considered an example of TA.
Catecholamines can cause DADs by causing intracellular Ca2+ overload via increased ICa-L and the Na+/Ca2+ exchange current.
Ischemia-induced DADs are believed to be caused by the buildup of lysophosphoglycerides in the ischemic myocyte, leading to an increase in Na+ and Ca2+.
Adenosine indirectly reduces the Ca2+ inward current by inhibiting the effects on adenylate cyclase and cyclic adenosine monophosphate. As a result, it may eliminate DADs induced by catecholamines but does not affect DADs induced by Na+ /K+ pump inhibition.
Examples include atrial tachycardia, digitalis toxicity-induced tachycardia, accelerated ventricular rhythms in the setting of acute myocardial infarction, some forms of repetitive monomorphic VT, reperfusion-induced arrhythmias, right ventricular outflow tract VT, and exercise-induced VT (e.g., catecholaminergic polymorphic VT).
Early after depolarization
The EADs are oscillatory potentials that occur during the AP plateau (phase 2 EADs) or the late repolarization (phase 3 EADs).
Phase 2 EADs are related to Ica-L current, while phase 3 EADs may result from electronic current during repolarization or low IK1.
EADs develop from action potential prolongation(AP), manifesting as QT prolongation on the surface electrocardiogram (ECG). Various agents and conditions can lead to decreased outward or increased inward current, shifting the normal outward current and creating the necessary conditions for EADs.
Hypokalemia and/or bradycardia are additional factors that result in the prolongation of the AP.
An EAD-mediated TA appears to be the underlying cause of arrhythmias that develop in the setting of long QT syndrome.
Early afterdepolarization-triggered arrhythmias depend on heart rate, and generally, the EAD amplitude increases at slower heart rates. A prolonged compensatory pause following a premature stimulus can be more important than bradycardia in initiating torsades de pointes.
References
1. Gaztañaga L, Marchlinski FE, Betensky BP. Mechanisms of Cardiac Arrhythmias. Revista Española de Cardiología (English Edition). 2012;65(2):113–201.
