Hypoxemic Respiratory Failure in Critically Ill Cardiac Patients
Causes of Hypoxemia
1. Anatomical Right-to-Left Intracardiac Shunt: Conditions such as Atrial Septal Defect (ASD) or Ventricular Septal Defect (VSD) can lead to mismatched ventilation and perfusion.
Invasive Mechanical Ventilation in the CICUs
-
The systemic inflammatory cascade is accompanied by the upregulation of inflammatory cytokines, including interleukin (IL)-1β, IL-6, IL-8, and tissue necrosis factor-α, as well as the activation of the complement system. This inflammatory response may also increase the permeability of the pulmonary vascular membrane, resulting in noncardiogenic pulmonary edema.
-
A rise in left ventricular end-diastolic and left atrial pressures increases hydrostatic pressures in the pulmonary capillaries. Elevated hydrostatic pressures overwhelm the active lymphatic resorption of fluids, leading to alveolar edema and impaired gas exchange. Strategies for mechanical circulatory support that increase end-diastolic pressure can also enhance this mechanism.
-
The consequences of high filling pressures and capillary leak in cardiogenic shock may lead to worsening hypoxemia, which can cause further myocardial ischemia, tissue hypoxia, and progressive multiorgan failure.
Heart-Lung Interaction During Invasive Mechanical Ventilation
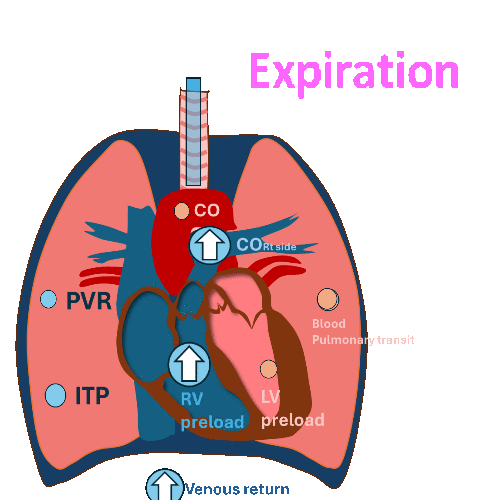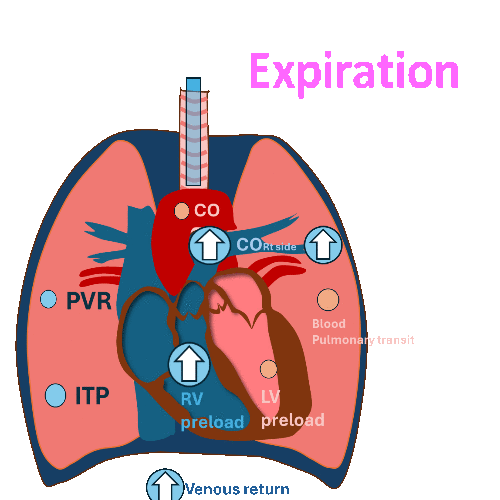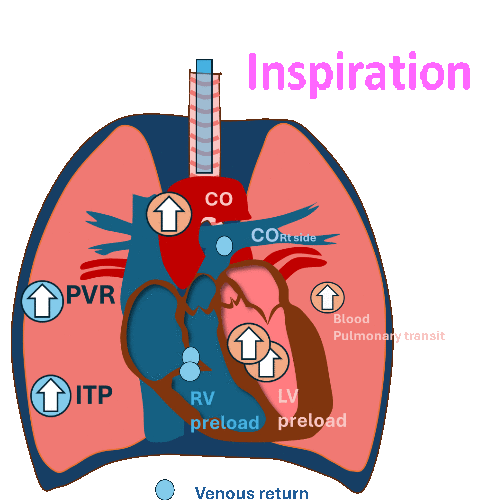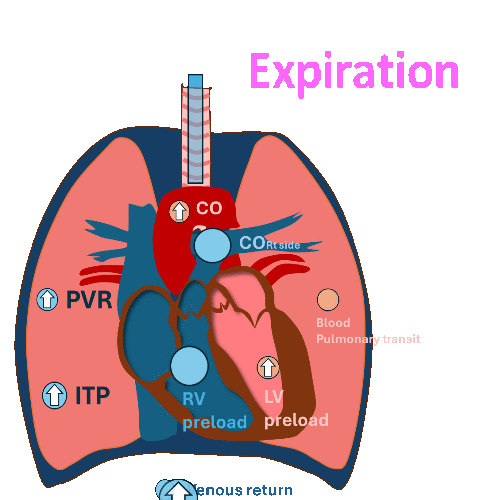
During Invasive mechanical ventilation
Inspiration
Decrease RV preload and a slight increase in RV afterload.
Decrease RV stroke volume.
Pulmonary transit
Expiration
Decrease left ventricular (LV) filling and
decrease left ventricular stroke volume at expiration.
(RV output during inspiration = LV filling during expiration)
PVR: Pulmonary vascular resistance, ITP: Intrathoracic pressure, LV: left ventricle, RV, right ventricle, CO: cardiac output
Hemodynamic changes have different effects on the right and left sides of the heart.
Effects on Right Ventricle
RV preload equals cardiac venous return. Venous return is primarily determined by the difference between mean systemic filling pressures and right atrial pressure (mean filling pressure - RAP).
Initiating invasive mechanical ventilation raises intrathoracic pressure, which in turn elevates right atrial pressure. This change reduces the gradient between mean systemic filling pressures and the right atrium, consequently impairing venous return and leading to a decline in right ventricular preload.

Boron & Boulpaep: Medical Physiology and West Respiratory Physiology
Invasive Mechanical ventilation significantly affects right ventricular (RV) afterload, typically described by a U-shaped curve that is mediated by changes in lung volume and pulmonary vascular resistance.
PEEP may help reduce atelectasis and, for some patients, decrease right ventricular (RV) afterload. However, in most cases, invasive mechanical ventilation increases RV afterload.
The combination of lower right ventricular preload and higher right ventricular afterload leads to a decrease in cardiac output on the right side.
PEEP application in patients with RV dysfunction requires a balanced approach: PEEP can lower RV afterload by avoiding atelectasis and improving oxygenation; however, excessive PEEP raises intrathoracic pressure, impairing RV preload and output.
PEEP must be carefully titrated to each patient’s lung mechanics and hemodynamic status to balance alveolar recruitment with RV performance.
Patients with preserved right ventricular (RV) function and adequate preload generally show less sensitivity to positive end-expiratory pressure (PEEP).
However, caution is required when adjusting PEEP in individuals experiencing refractory hypoxemia or acidosis, as these conditions can lead to an increase in right ventricular afterload. In patients with interstitial lung disease, even small changes in alveolar pressure can significantly increase right ventricular afterload.
For those with right ventricular preload-dependent conditions—especially individuals with right ventricular dysfunction or pulmonary hypertension—avoiding intubation is ideal whenever possible. If intubation becomes necessary, the endotracheal tube should be set to the lowest effective level of positive end-expiratory pressure (PEEP) to ensure adequate oxygenation while preventing lung atelectasis.
In systemically hypotensive patients, increasing PEEP can raise central venous pressures, decrease right ventricular perfusion pressure, and exacerbate right ventricular ischemia.
Effects on the Left Ventricle
In the absence of intracardiac shunts, left ventricular (LV) preload is determined by the cardiac output from the right ventricle (RV). Considering the hemodynamic effects of mechanical ventilation on the RV, there is often a decrease in LV preload.
PEEP during mechanical ventilation reduces left ventricular (LV) afterload through several mechanisms.
-
PEEP increases pressure within the left ventricle and the thoracic aorta, creating a pressure gradient between intrathoracic structures and the systemic circulation. This gradient promotes forward blood flow.
-
Additionally, the elevated intrathoracic pressures stimulate aortic baroreceptors, which leads to a reduction in systemic vascular resistance.
-
Furthermore, positive pleural pressures decrease the transmural pressure of the left ventricle, thereby reducing LV wall tension.
Patients who are predominantly afterload-sensitive, most commonly those with left ventricular (LV) dysfunction, may experience beneficial effects when higher positive end-expiratory pressure (PEEP) is used.
Low tidal volume ventilation
Pathophysiologically, volutrauma, atelectotrauma, and barotrauma all contribute to elevated levels of inflammatory cytokines, which can further exacerbate multi-organ failure. The goal of lung protective ventilation is to minimize the risk of ventilator-associated lung injury and diaphragm damage. However, the effectiveness of low tidal volume ventilation in cases of acute hypoxemic respiratory failure not caused by acute respiratory distress syndrome (ARDS) remains uncertain. The PEEP strategies have been discussed above.
"Effect of a Low vs Intermediate Tidal Volume Strategy on Ventilator-Free Days in Intensive Care Unit Patients Without ARDS" was a randomized clinical trial conducted from September 1, 2014, to August 20, 2017, in six ICUs in the Netherlands. The study involved patients with ARDS who were expected to remain on ventilation for at least 24 hours, with participants receiving either low tidal volumes (n = 477) or intermediate tidal volumes (n = 484). The primary outcome was the number of ventilator-free days alive at day 28.
The results indicated that the low tidal volume strategy did not result in more ventilator-free days compared to the intermediate tidal volume strategy among ICU patients without acute respiratory distress syndrome (ARDS).
Another major cause of ventilator-associated lung injury is patient-ventilator dyssynchrony. Asynchrony can occur at any stage of breath triggering, delivery, and termination. Patient-ventilator dyssynchrony is associated with worse clinical outcomes and can contribute to ongoing lung injury. Strategies to minimize dyssynchrony include adjusting ventilation parameters to match patient demand, optimizing sedation, and, in some cases, administering paralysis.
Refractory hypoxemia
Even with optimization efforts, some patients in the Cardiac Intensive Care Units (CICUs) may experience refractory hypoxemia. This condition is characterized by a partial pressure of arterial oxygen (PaO2) of 60 mmHg or lower, or a specific PaO2-to-fraction of inspired oxygen (FiO2) ratio of 100 or less when the FiO2 is 0.8 or higher. Additionally, it can involve using positive end-expiratory pressure (PEEP) greater than 15 cm H2O or plateau pressures exceeding 30 cm H2O for more than 12 hours, despite the use of low tidal volume ventilation (LTVV).
Management of refractory hypoxemia may include additional treatment strategies such as prone positioning, neuromuscular blockade, inhaled pulmonary vasodilators (such as nitric oxide or epoprostenol), inverse inspiratory/expiratory (I/E) ventilation, and extracorporeal membrane oxygenation (ECMO). There is a knowledge gap regarding the use of these adjunctive treatments for refractory acute hypoxemic respiratory failure (AHRF) in patients in the cardiac intensive care unit (CICU).
PRONE POSITIONING. Prone positioning for at least 16 hours a day has been shown to reduce mortality in patients with moderate to severe acute respiratory distress syndrome (ARDS). This positioning can improve right ventricular (RV) hemodynamics and may be beneficial in cases of acute cor pulmonale resulting from acute hypoxic respiratory failure (AHRF). However, the hemodynamic effects on the left ventricle (LV) are unclear.
Ventricular arrhythmias continue to be a major concern for patients in cardiac intensive care units (CICUs). Even under ideal circumstances, repositioning patients to facilitate chest compressions can take up to three minutes.
NEUROMUSCULAR BLOCKADE. Neuromuscular blockade in patients with severe ARDS is believed to improve outcomes by relaxing the expiratory muscles, reducing patient-ventilator dyssynchrony, lowering the work of breathing, and potentially providing anti-inflammatory effects. However, the use of paralysis is associated with several significant adverse effects, including myopathy, polyneuropathy, pressure ulcers, deep venous thrombosis, and corneal abrasions.
Neuromuscular blockade is primarily used in patients who are dyssynchronous with the ventilator despite adequate sedation or those with severe hypoxemia. Although neuromuscular blocking agents generally do not have direct hemodynamic effects, they may lower myocardial oxygen demand.
Guidelines conditionally recommend the use of neuromuscular blockade in the early stages of severe acute respiratory distress syndrome (ARDS). The ACURASYS trial, which examined neuromuscular blockers in early ARDS, showed an improvement in 90-day survival rates. In contrast, the ROSE trial, which focused on early neuromuscular blockade with a lighter sedation strategy, did not demonstrate any benefits.
INHALED NITRIC OXIDE AND PULMONARY VASODILATORS. Inhaled nitric oxide (iNO) may improve V/Q mismatch via local pulmonary vasodilation in well-ventilated alveoli. While iNO often improves oxygenation, there is no established reduction in mortality..
In patients with cardiogenic shock (CS) who experience primarily right ventricular (RV) failure, inhaled nitric oxide (iNO) may play a role in temporarily reducing pulmonary vascular resistance and enhancing cardiac index. Conversely, in patients with significant left ventricular (LV) dysfunction, an increase in right-sided cardiac output may elevate LV preload.
EXTRACORPOREAL MEMBRANE OXYGENATION. V-V ECMO (veno-venous extracorporeal membrane oxygenation) has been shown to reduce mortality in patients with severe ARDS (acute respiratory distress syndrome). It may also indirectly improve right ventricular (RV) hemodynamics by enabling ultra-lung protective ventilation and enhancing hypoxic vasoconstriction. However, if ECMO is being considered for patients with left ventricular (LV) predominant cardiogenic shock (CS) and refractory hypoxemia, a V-A (veno-arterial) configuration would be more appropriate.
Weaning From Invasive Mechanical Ventilation
Several criteria help determine if a patient is ready for extubation:
1. Resolution of the condition that led to intubation,
2. Absence of significant tracheal secretions,
3. An adequate cough reflex and mental status,
4. A stable cardiovascular and respiratory condition.
Implementing standardized breathing trials can shorten the overall duration of mechanical ventilation and reveal both objective and subjective indicators of potential failure.
Weaning-induced pulmonary edema is a common reason for weaning failure, so it is crucial to optimize the patient's volume status before proceeding with extubation.
Other important factors that can lead to failed Spontaneous Breathing Trials (SBTs) include excessive respiratory load, neuromuscular dysfunction, critical illness, neuropsychological factors, and metabolic or endocrine disorders.
References
1) Jozwiak M, Teboul JL. Heart-Lungs interactions: the basics and clinical implications. Ann Intensive Care. 2024 Aug 12;14(1):122. doi: 10.1186/s13613-024-01356-5.
2) Sterling LH, Fernando SM, Lawler PR, Price S, Fan E, Goligher E, et al. Navigating Hypoxemic Respiratory Failure in Critically Ill Cardiac Patients. JACC: Advances [Internet]. 2025 Apr 16;101616.
3) Papazian Laurent, Forel Jean-Marie, Gacouin Arnaud, Penot-Ragon Christine, Perrin Gilles, Loundou Anderson, et al. Neuromuscular Blockers in Early Acute Respiratory Distress Syndrome. New England Journal of Medicine [Internet]. [cited 2025 Apr 28];363(12):1107–16.
4) the PReVENT Investigators. Effect of a Low vs Intermediate Tidal Volume Strategy on Ventilator-Free Days in Intensive Care Unit Patients Without ARDS: A Randomized Clinical Trial. JAMA. 2018;320(18):1872–1880.
5) National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, Grissom CK, Gundel S, Hayden D, Hite RD, Hou PC, Hough CL, Iwashyna TJ, Khan A, Liu KD, Talmor D, Thompson BT, Ulysse CA, Yealy DM, Angus DC. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N Engl J Med. 2019 May 23;380(21):1997-2008.
